We introduce an efficient element based reduction technique for molar formulation. The chemical equilibrium constraints are included into the multiphase multi-component flash calculation which solves the thermodynamic and chemical phase equilibrium simultaneously13. This provides a generic treatment of chemical and thermodynamic equilibrium within the successive substitution loop of multiphase flash to accommodate chemical precipitation and dissolution. Using the Equilibrium Rate Annihilation matrix allows us to reduce the governing unknowns to the element conservation equations while the coupling between chemical and thermodynamic equilibrium is captured by modified multiphase flash equations.
A finite-volume unstructured discretization in space is applied together with a fully-implicit approximation in time. The resulting complex nonlinear system is parameterized using the OBL approach. In OBL, the time-consuming phase and chemical equilibrium computations are significantly decreased which reduce linearization time and the number of nonlinear iterations. Besides, equilibrium reactions are fully coupled with kinetic reactions in DARTS. Fig.12 presents several examples where equilibrium reactions describing the dissolution in carbonates are compared with the kinetic reactions at different kinetic rates.
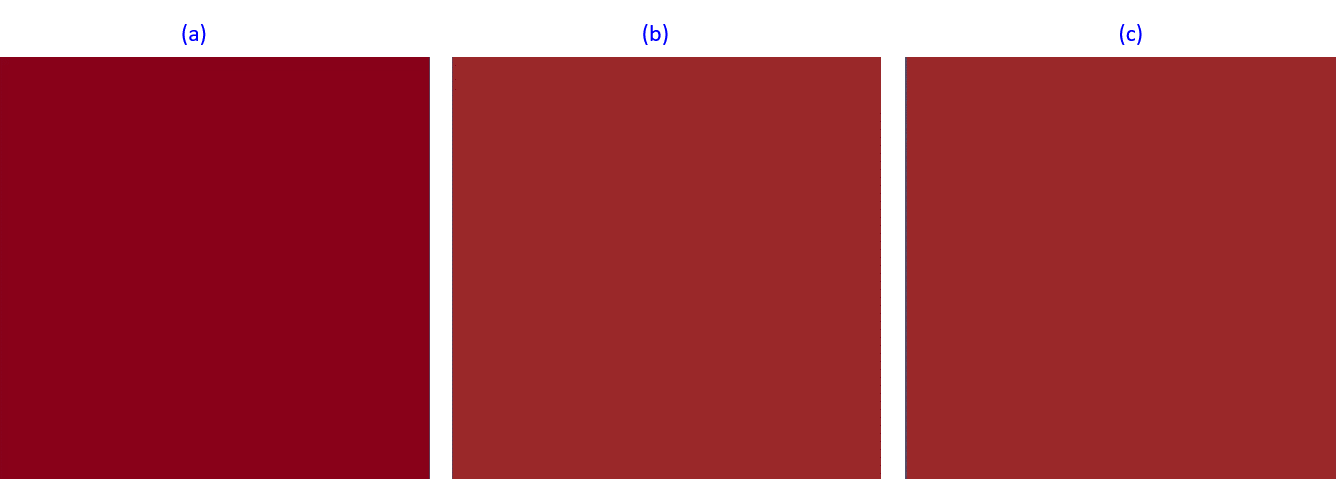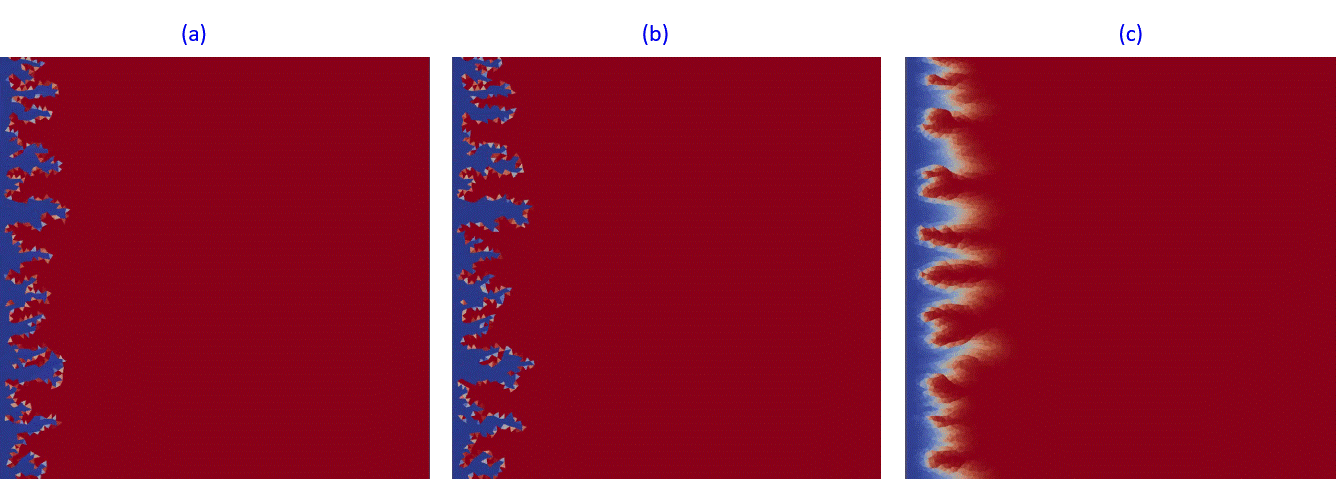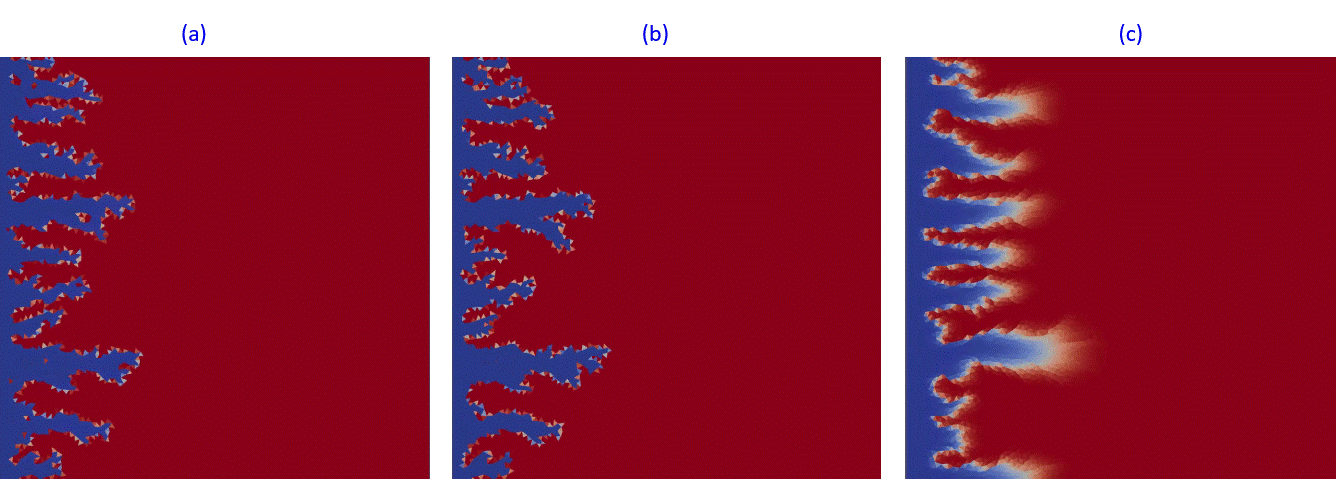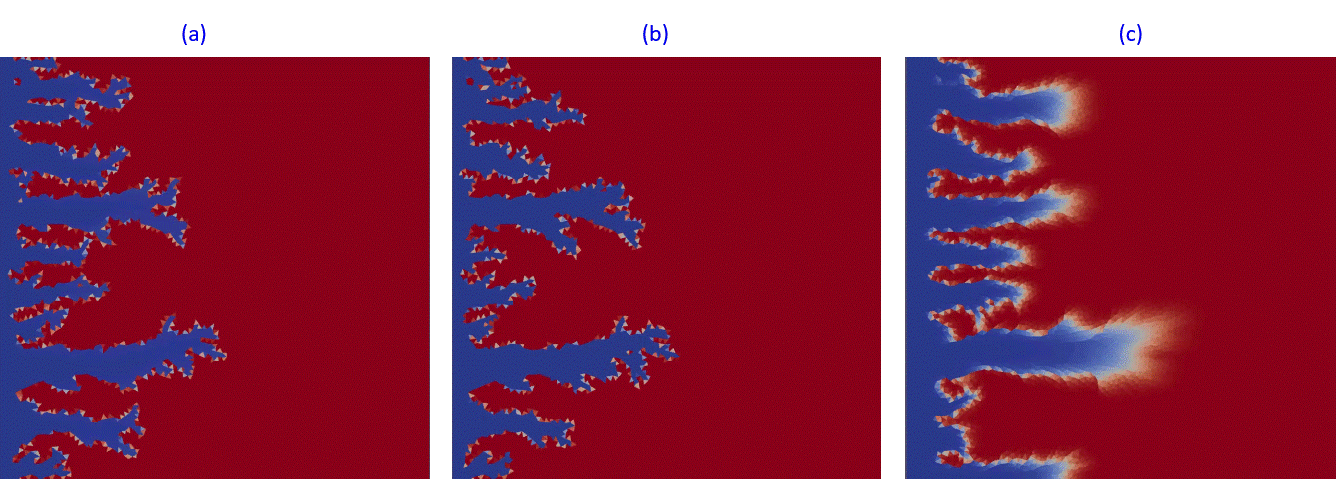
Fig.12: Dissolution with equilibrium (a), high-rate (Da~10) kinetic (b) and lower rate (Da~0.001) kinetic reactions.
Another example in Fig.13 presents kinetic dissolution in the complex fractured system at different aperture distribution decreasing from (a) to (c). In Fig.13,a, the acidic fluid is propagated by fracture system to the right border while in Fig.13,c, fractured system is almost closed and plays the role of permeability perturbation.
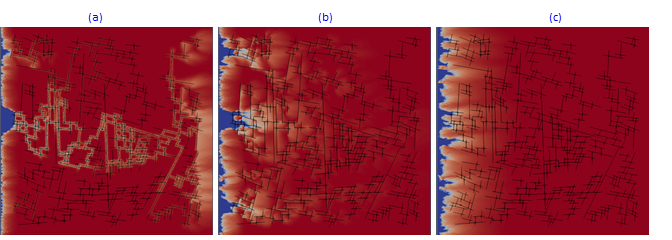
Fig.13: Kinetic dissolution in fractured reservoir with fractures apperture changing from 1 mm (a) to 0.5 mm (b) and finally to a fully closed system (c).
