Geological storage of CO2 is a crucial and upcoming technology to reduce anthropogenic greenhouse gas emissions. Due to the buoyant characteristic of injected gas and the complex geology of subsurface reservoirs, the security of underground storage is a major concern. To assess the security of CO2 storage, an accurate prediction of the CO2 plume migration is essential. In this study, an accurate thermodynamic model is developed to describe realistic gas behaviour in a saline aquifer and to understand the factors affecting CO2 storage. A recently developed thermodynamic model is based on a combination of Peng-Robinson10 and Kritchevsky-Illiinskaya11 equations of state. This model combines classic fugacity-based formulation for the supercritical gas phase and activity model combined with Henry’s constants for the aqueous brine.
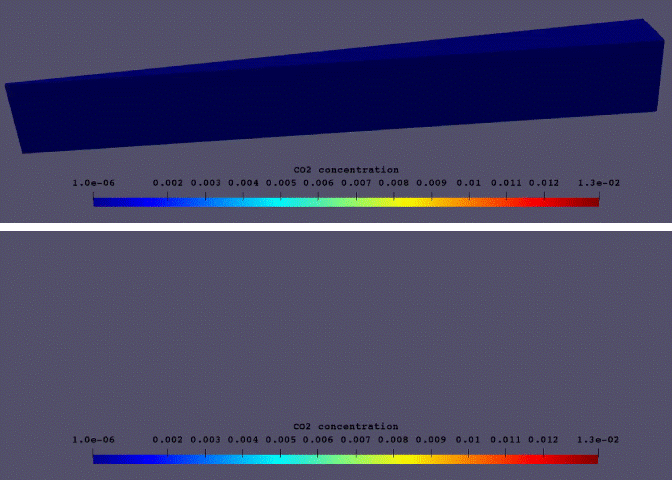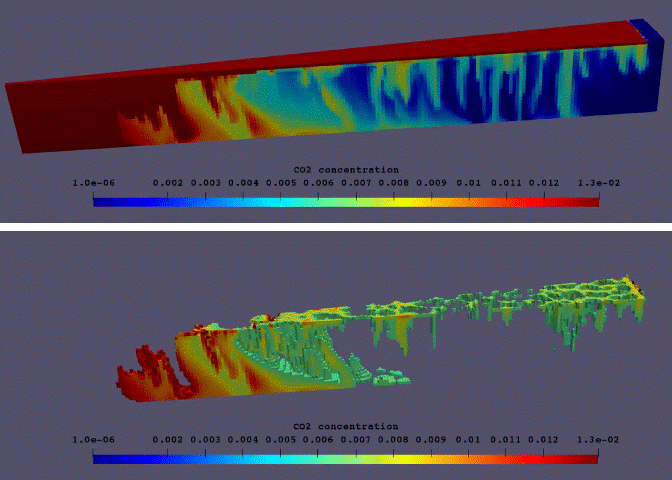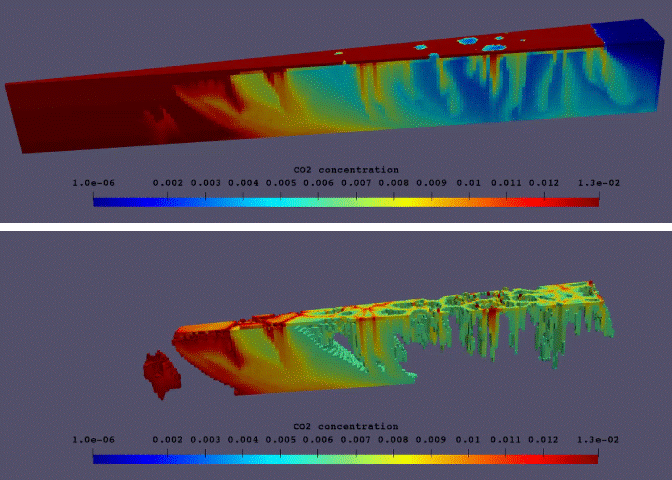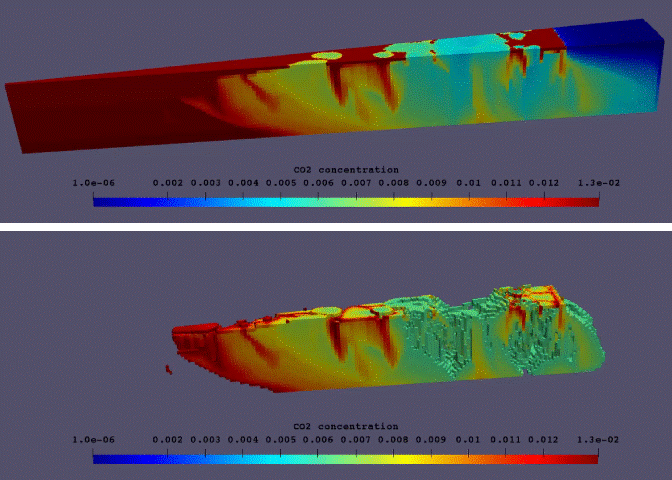
Fig.11: Supercritical CO2 dissolution in realistic 3D model of aqueous aquifer.
The phase behaviour based on this thermodynamic model and a consistent set of physical properties has been implemented in DARTS. An example of an enhanced dissolution of supercritical CO2 in a geologic aquifer is illustrated by an unstructured 3D model shown in Fig.11. The model can accurately capture a phase behaviour in supercritical CO2-brine-impurity system and predict an enhanced dissolution rate for the variety of changing parameters including pressure, temperature and salinity of brine. The enhanced dissolution rate in realistic 3D conditions is different from its 2D analogue. This model can be fully coupled with chemical reaction relevant to CO2 sequestration12 for longer time-scale predictions.
